Cell Potential Formula
E 0cell refers to standard cell potential. The answer options are A 1241 volts B 1755.

Cell Potential Problems Electrochemistry Youtube
R is the gas constant 83145 JmolK T is.

. Tin is oxidized at the anode while silver ion is reduced at the cathode. E cell E 0cell - RTnF x lnQ. The Standard Cell Potential eqEcirc eq.
The electrical potential between the electrodes of a cell can be calculated from tabulated standard half-cell potentials. The difference in potential energy between the anode and cathode is known as the cell potential in a voltaic cell. Ni 2 2 e- Ni E 0 -025 V Pb 2 2 e-.
The result is the. The formula for cell potential is. The cell would therefore proceed spontaneously in Case 2Notice that we did not multiply the value for the.
The highest positive potential is found by using the Zr oxidation half-reaction. You can calculate the cell potential for an electrochemical cell from the half-reactions and the operating conditions. EMF of a Cell.
The standard cell potential or standard electromotive force emf is the difference between the electrode potential reduction potential of the cathode and anode. The Nernst equation is. E cell is the cell potential.
Example Find the cell potential of a galvanic cell based on the following reduction half-reactions where Ni 2 0030 M and Pb 2 0300 M. Note that the voltage for. The difference in potential between two half cells in an electrochemical cell is called the cell potential Ecell.
It can also be defined as the net voltage between. The first step is to. The standard cell potential is positive so the reaction is spontaneous as written.
If the value of delta G is positive the. The potential difference is caused by the. Because the actual cell potential E is compared with the maximum possible cell potential E r allowed by the second law the voltage efficiency is really a specific form of the exergy.
The electromotive force of a cell or EMF of a cell is the maximum potential difference between two electrodes of a cell. In this formula n refers to the number of moles for the substance F stands for Faradays constant and E is the standard cell potential. Ni 2 2 e- Ni E 0 -025 V Pb 2 2 e-.
The other half-equation is Ni2 aqueous plus two electrons giving Ni solid with its standard electrode potential of negative 0257 volts. Example Find the cell potential of a galvanic cell based on the following reduction half-reactions where Ni 2 0030 M and Pb 2 0300 M.

Electrochemistry Calculating Cell Potential Chemistry Stack Exchange

19 1 Calculating Cell Potential Hl Youtube
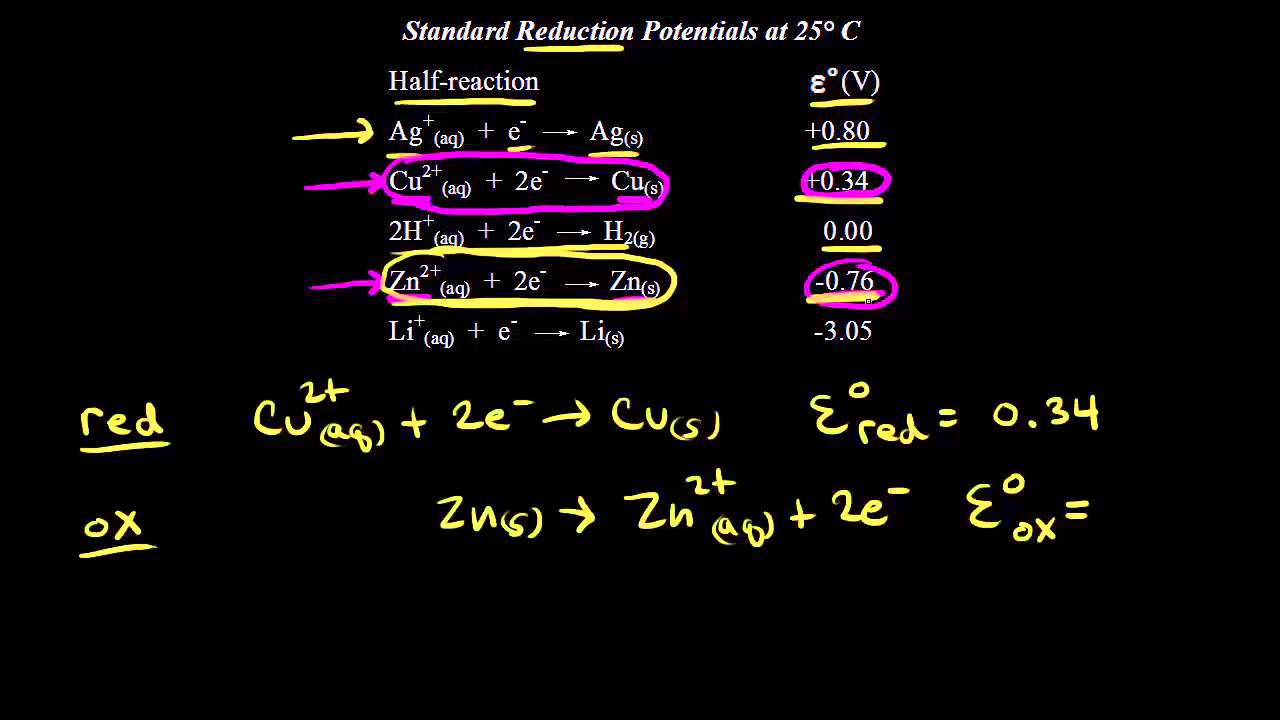
Standard Reduction Potentials Video Khan Academy

Question Video Calculating The Standard Cell Potential For A Gold Nickel Cell Nagwa
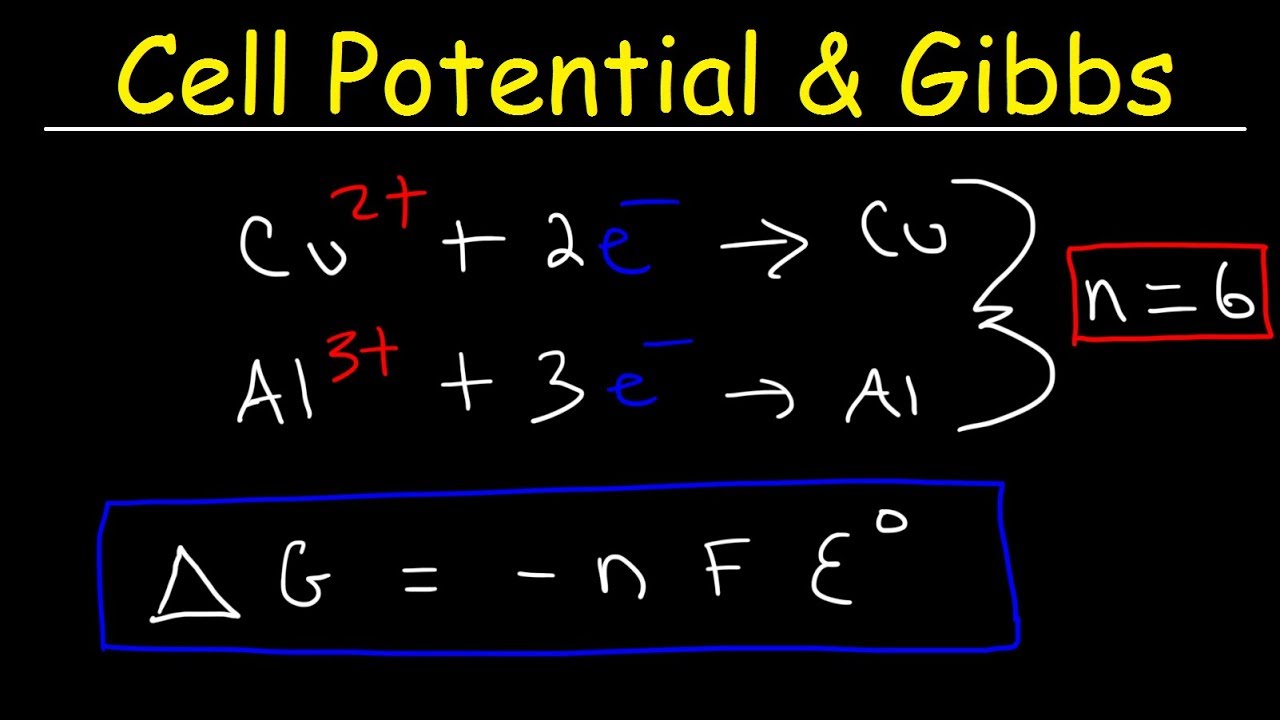
Cell Potential Gibbs Free Energy Standard Reduction Potentials Electrochemistry Problems Youtube

Question Video Calculating A Cell Potential From Standard Electrode Potentials Of Cadmium And Nickel Nagwa
0 Response to "Cell Potential Formula"
Post a Comment